The Expanding Roles of PARP Inhibitors in Prostate Cancer
Nicholas G. Little, PharmD
PGY-2 Oncology Pharmacy Resident
Southern Arizona VA Health Care System
Tucson, Arizona
Introduction
Over the last decade, the role of targeted therapy has played a key role in shaping clinical practice for many different malignancies. Despite being the most common cancer among men, there has been very little development of targeted therapy in prostate cancer.1 This could be contributed in part to the effectiveness of androgen pathway therapies. Unfortunately, these agents typically are not curative, and the development of resistance is not uncommon. It's for this reason that investigating new novel treatment modalities are so important. One class of medication that has found a niche for itself in prostate cancer are the Poly ADP-ribose polymerase (PARP) inhibitors.
Poly ADP-ribose polymerase is a class of enzymes involved in DNA transcription, cell cycle regulation, and DNA repair.2 Several malignancies can present from what is known as homologous recombinant repair mutations (HRRm). These mutations result in DNA repair, which increases the likelihood of carcinogenesis. Roughly 20% of prostate cancer cases have been found to be positive for one of these mutations, and it is typically associated with worse outcomes.3 PARP inhibitors target this pathway and have been found to be an effective method of managing disease.
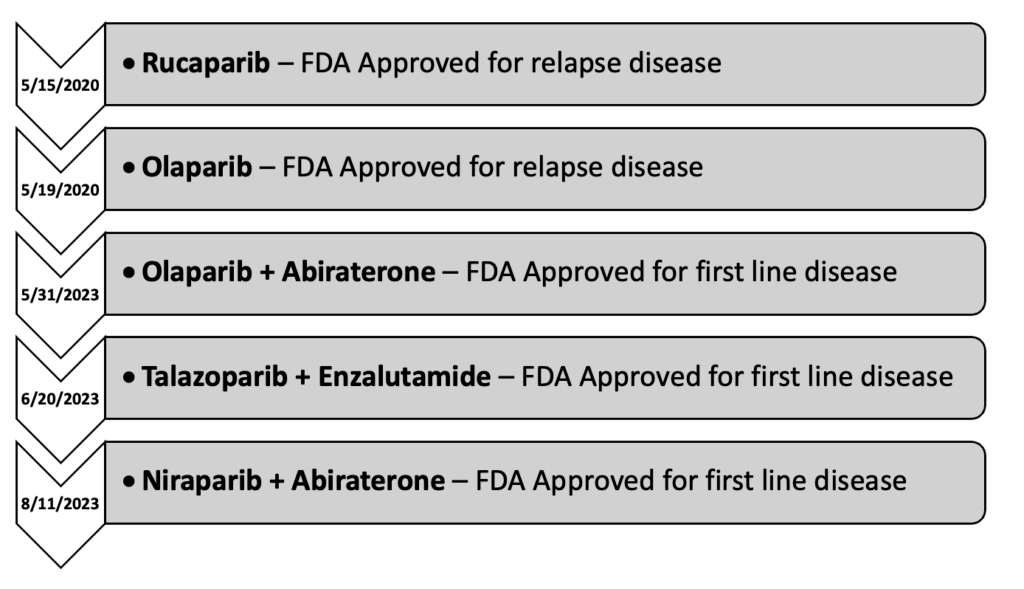
Use of these agents in prostate cancer initially started as monotherapy following prior lines of therapy in patients with metastatic castration resistant prostate cancer (mCRPC). However, with the conclusion of several trials in 2023, the role has expanded into the first line setting as part of a combination regimen in patients with mCRPC. Currently, there are four PARP inhibitors approved for use in prostate cancer: olaparib, rucaparib, talazoparib, and niraparib.
Timeline of PARP Inhibitors in mCRPC
Olaparib
Originally approved on 12/19/2014 for advanced stage ovarian cancer, olaparib didn't see use in prostate cancer until many years later with the conclusion of the PROfound trial.4 This phase III study was initially published on 5/28/2020 and evaluated the safety and efficacy of olaparib in men with metastatic castrate resistant prostate cancer (mCRPC) who had received prior treatment with either abiraterone or enzalutamide, prior taxane use was allowed. The subjects received either olaparib 300 mg twice daily or physicians' choice of abiraterone or enzalutamide. The subjects were further broken into two cohorts: cohort A and cohort B. Cohort A included subjects with BRCA1/2 or ATM mutations, while cohort B contained subjects with other identified DMR mutations. This trial found that olaparib doubled progression free survival (7.4 vs 3.6 months HR 0.34; 95% CI 0.25-0.47; P<0.001) and increased overall survival by about 3-months (18.5 vs 15.1 months HR 0.64; 95% CI 0.43-0.97; P=0.02) compared to the other treatment groups. Despite these promising results, the subgroup analysis found that the benefit was driven primarily by subjects with BRCA1/2 mutations, with BRCA2 having the most clear benefit. However, olaparib was not without toxicity. The incidence of grade ≥3 adverse events (AE) was 51%, with the most common grade ≥3 being anemia (21%). It should also be noted that almost half of all subjects (45%) had to have dose interruptions due to AE. A further 22% required dose reductions, and another 18% had that agent discontinued due to AE. The results of this trial ultimately led to the approval of olaparib on 5/19/2020.5 Later that year, on 12/20/2020, the final OS data was published.6 This final analysis demonstrated an OS increase of about 5-months in cohort A (19.1 vs 14.7 months HR 0.69; 95% CI 0.50-0.97; P=0.02).
Rucaparib
The same year that olaparib was approved, another PARP inhibitor, rucaparib, was studied for use in prostate cancer. Prior to this study, rucaparib was primarily used in ovarian and other gynecologic malignancies. The use of it in prostate cancer was first established with the publication of the phase II TRITON2 trial, on 11/10/2020.7 This study investigated the use of rucaparib in men with mCRPC who had progressed on both second-generation antiandrogens and taxane-based therapy. All subjects had either BRCA1/2 or another HRR mutation. The subjects received rucaparib 600mg twice daily. This trial demonstrated that rucaparib had an objective response rate (ORR) of 43.5% and a median PFS of 8.5-months. Grade ≥3 AE were fairly common (60.9%), with the most common grade ≥3 AE being anemia (25.2%), thrombocytopenia (9.6%), and fatigue (8.7%). Similar to what was seen with olaparib, treatment interruptions and dose reductions due to AE were common (56.5%)(40.9%). The results of this study lead to the accelerated approval of rucaparib on 5/15/2020.8
Rucaparib remained as a subsequent line of therapy for another three years, until the publication of the TRITON3 trial on 2/23/2023.9 This study was the phase III continuation of the TRITON2 trial; investigating the safety and efficiency of rucaparib in mCRPC in subjects BRCA or ATM mutations who had progression on prior second-generation androgen receptor pathway inhibitors. Subjects either got rucaparib or either second generation androgen receptor pathway inhibitor or taxane-based chemotherapy. The results of this trial showed a benefit in terms of PFS for the BRCA subgroup (11.2 vs 6.4 months HR 0.50; 95% CI 0.36-0.69; P<0.001), however, no appreciable difference was seen in terms of OS. Additionally, no benefit was seen in the BRAC1 and ATM groups. Rates of AE were similar to what was seen in the TRITON2 trial.
Niraparib + Abiraterone
The TRITON3 trial marked the end of trials investigating single agent PARP inhibitors in prostate cancer. From here, the focus shifted to combination regimens. The MAGNITUDE trial was the first large scale study evaluating the use of a PARP inhibitor in combination with another therapeutic agent for prostate cancer.10 This study investigated the use of niraparib, another PARP inhibitor, in combination with abiraterone in men with HRRm positive mCRPC. Prior use of abiraterone or second-generation antiandrogens was permitted. Subject were randomized to either get niraparib 200mg daily with abiraterone or abiraterone with a matched placebo. The initial analysis demonstrated a modest PFS improvement in patients with HRRm (16.5 vs 13.7 months HR 0.73; 95% CI 0.56-0.96; P=0.022), with the most benefit seen in the BRCA1/2 subgroup (16.6 vs 10.9 months HR 0.53; 95% CI 0.36-0.79; P=0.001). Similar results were seen in the second interim analysis for PFS, unfortunately no OS benefit was demonstrated.11 Rates of grade ≥3 toxicity were about 20% higher with the combination (67.0% vs 46.4%), with the most prominent being anemia (28.3% vs 7.6%). Around 20% of patients required dose reduction of niraparib due to toxicity, with an additional 10% requiring permanent discontinuation. Based on the results of this study, the FDA approved the combination of niraparib/abiraterone on 8/11/2023.
Olaparib + Abiraterone
Around the same time as the MAGNITUDE trial, the PROpel trial was investigating a similar regimen utilizing olaparib.12 This phase III trial compared the use of olaparib with abiraterone to abiraterone and a matched placebo in all patients regardless of HRRm status. The dosing of olaparib and abiraterone were 300mg twice daily and 1000mg daily respectively. Surprisingly, initial results indicated that there may be a PFS benefit regardless of HRRm status. In patients with HRRm, PFS was improved by about 15-months (28.8 vs 13.8 months HR 0.45; 95% CI 0.31-0.65), and about 8-months in patients without HRRm (27.6 vs 19.1 months HR 0.72; 95% CI 0.56-0.93). However, these hopes were quickly dashed with the publication of the final OS results a year later.13 Not only did data show a lack of survival benefit in patients without HRRm, there was also a lack in benefit in the HRRm groups. Only once the HRRm was further broken down into BRCA and non-BRCA mutations do we see that the true benefit is only present in patients with BRCAm disease (NR vs 23.0 months HR 0.29; 95% CI 0.14-0.56). The results of this study further support what was established in the PROfound trial: that the benefit of olaparib is most prominent in the BRCAm mutations. Unfortunately, the difference in BRCA1 and BRCA2 was not reported in the PROpel, so it's unclear if there's any difference in that regard. Rates of grade ≥3 toxicities were about 10% higher in the combination group (47.2% vs 38.4%). Similar to other trials, the most common grade ≥3 event was anemia (15.1% vs 3.3%). Overall rates of olaparib discontinuation were low (13.8% vs 7.8%). On 5/31/2023, the FDA labeling for olaparib was updated to reflect this trial.
While this combination was approved for in the first line setting for mCRPC, some of the study design choices are concerning and may make clinical application more difficult. This is because the PROpel study excluded patients with prior use of abiraterone or second-generation antiandrogens. Because guideline directed therapy for metastatic castrate sensitive disease (mCSPC) heavily rely on these agents early in therapy, the bulk of patients who progress from castrate sensitive disease were not represented by this study.14
Talazoparib + Enzalutamide
The most recent study to come out on this subject is the TALAPRO-2 trial.15 Originally published on 7/22/2023, this phase III trial investigated the use of talazoparib with enzalutamide vs enzalutamide with placebo in patients with previously untreated mCRPC. Prior use of abiraterone was permitted, however prior use of second-generation antiandrogens (enzalutamide, darolutamide, apalutamide) were excluded. Patients were included regardless of HRRm status. In the initial analysis, the primary endpoint was only reached in HRRm positive group, which demonstrated an improvement in PFS of about 11 months (27.9 vs 16.4 months HR 0.46; 95% CI 0.30-0.70; P=0.0003). Interestingly, these initial results indicate that there may be a PFS benefit in non-HRRm patients (NR vs 22.5 months HR 0.70; 95% CI 0.54-0.89; P=0.0039), however this will be determined by subsequent follow up. This combination was not without toxicity. Overall rates of grade ≥3 toxicity were significantly higher in the talazoparib group (75% vs 45%). Similar to the other studies, anemia was the most common grade ≥3 toxicity (46%). Additionally, dose interruptions and reductions were required in over 50% of patients (62% and 53%, respectively). Based on the results of this data, on 6/20/2023, the FDA approved the use of talazoparib with enzalutamide as first line therapy for mCRPC in patients with HRRm.16
Summary
| Regimen | Place in therapy | Comparator | Outcomes |
| Olaparib | Subsequent Line mCRPC | Abiraterone or Enzalutamide | BCRA1/2, ATM
OS: 19.1 vs 14.7 months (HR 0.69, 95% CI 0.50-0.97; p=0.02) |
| Rucaparib | Subsequent Line mCRPC | Abiraterone, Enzalutamide, or Docetaxel | BCRA1/2, ATM
PFS: 10.2 vs 6.4 months (HR 0.61, 95% CI 0.47-0.80; p<0.001) |
| Niraparib + Abiraterone | First Line mCRPC | Abiraterone | BRCA1/2
PFS: 19.5 vs 10.9 months (HR 0.55, 95% CI 0.39-0.78; p=0.0007) |
| Olaparib + Abiraterone | First Line mCRPC | Abiraterone | BRCA1/2
OS: NR vs 23.0 months (HR 0.29, 95% CI 0.14-0.56) |
| Talazoparib + Enzalutamide | First Line mCRPC | Enzalutamide | HRRm
PFS: 27.9 vs 16.4 months (HR 0.46, 95% CI 0.30-0.70; p=0.0003) |
The use of PARP inhibitors earlier in treatment emphasizes the importance of early testing. Guidelines by the National Comprehensive Cancer Network (NCCN) and the Veterans Health Administration (VHA) National Oncology Program provide recommendations on when testing for HRRm should be performed.14,17 Both guidelines agree that patients with high-risk/very high-risk localized, regional, and metastatic prostate cancer should be tested as part of the initial workup. As for their place in therapy; NCCN guidelines list niraparib with abiraterone and olaparib with abiraterone as a category 1 recommendation for patients with BRCA mutations and no prior novel hormone therapy or docetaxel.14 Talazoparib with enzalutamide has the same recommendation, but for patients with HRRm. Single agent olaparib is listed as a category 1 recommendation after progression on novel hormone therapy in patients with HRRm, whereas single agent rucaparib is listed as a category 2A recommendation after progression on novel hormone therapy in patients with BRCA mutation.
The prostate cancer clinical pathways, as endorsed by the VHA National Oncology Program, lists olaparib with abiraterone as the preferred first line option for patients with BRCA mutated mCRPC.17 Single agent olaparib is recommended in the second line setting for patients with HRRm, who would not be good candidate for docetaxel in addition to the third line setting for patients who would not be a good candidate for lutetium 177 and cabazitaxel. Olaparib is the only PARP inhibitor recommended by these guidelines and there are no recommendations regarding any of the other combination regimens.
Reference
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Rose M, Burgess JT, O'Byrne K, Richard DJ, Bolderson E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front Cell Dev Biol. 2020;8:564601. Published 2020 Sep 9. doi:10.3389/fcell.2020.564601
- Mandelker D, Zhang L, Kemel Y, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing [published correction appears in JAMA. 2018 Dec 11;320(22):2381]. JAMA. 2017;318(9):825-835. doi:10.1001/jama.2017.11137
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
- Center for Drug Evaluation and Research. FDA approves Olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. U.S. Food and Drug Administration. Accessed January 12, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383(24):2345-2357. doi:10.1056/NEJMoa2022485
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772. doi:10.1200/JCO.20.01035
- Center for Drug Evaluation and Research. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer . U.S. Food and Drug Administration. Accessed January 12, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician's Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388(8):719-732. doi:10.1056/NEJMoa2214676
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023;41(18):3339-3351. doi:10.1200/JCO.22.01649
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782. Doi:10.1016/j.annonc.2023.06.009
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid 2022;1(9). Doi: 10.1056/EVIDoa2200043
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094-1108. doi:10.1016/S1470-2045(23)00382-0
- NCCN Guidelines. Prostate Cancer. Version 4.2023. Updated 9/7/2023.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial [published correction appears in Lancet. 2023 Jul 22;402(10398):290]. Lancet. 2023;402(10398):291-303. doi:10.1016/S0140-6736(23)01055-3
- Center for Drug Evaluation and Research. FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. U.S. Food and Drug Administration. Accessed January 12, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate.
- US Department of Veterans Affairs. Oncology Clinical Pathways Prostate Cancer. Version 1.2024. Accessed January 12, 2024. https://www.cancer.va.gov/CANCER/assets/pdf/clinical-pathways/13/PrCCP.pdf
